Viclaner chewable tablets dewomer flurulaner dewomer chewables for dog use liver flavor
Indication:
It is used to treat flea and tick infection on the dog's body surface, and can also assist in the treatment of allergic dermatitis caused by fleas.
Validity Period:
24 months.
Assay Strength:
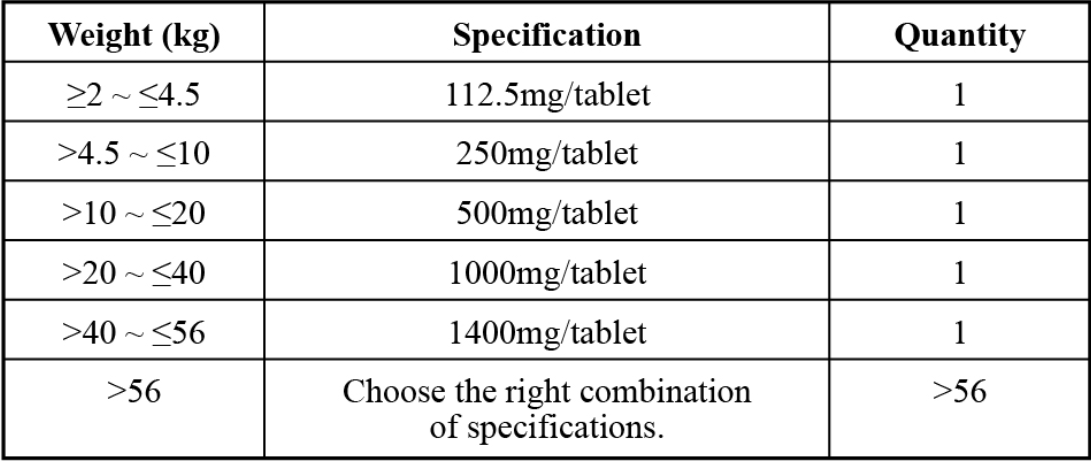
(1)112.5mg (2)250mg (3)500mg (4)1000mg (5)1400mg
Storage:
Sealed storage below 30℃.
Dosage
Cautions:
1. This product should not be used on puppies under 8 weeks old or dogs weighing less than 2kg.
2. Do not use in dogs allergic to this product.
3. The dosing interval of this product shall not be less than 8 week.
4.Do not eat, drink or smoke while administering the drug. Wash hands thoroughly with soap and water immediately after contact with this product.
5.Keep out of reach of children.
6.Please check whether the package is intact before use. If it is damaged, do not use it.
7.Unused veterinary drugs and packaging materials should be disposed of in accordance with local regulations.
Pharmacologic action:
Can be used for breeding dogs, pregnant and lactating female dogs.
Fluralaner has a high plasma protein binding rate and may compete with other drugs with a high protein binding rate, such as non-steroidal anti-inflammatory drugs, coumarin derivative warfarin, etc. In vitro plasma incubation tests, there was no evidence of competitive plasma protein binding between fluralaner and carprofen and warfarin. The clinical trials did not find any interaction between fluralaner and the daily medication used in dogs.
In case of any serious reactions or other adverse reactions not mentioned in this manual, please consult a veterinarian in time.
This product acts quickly and can reduce the risk of transmission of insect-borne diseases. But fleas and ticks must contact the host and start feeding in order to be exposed to the active drug ingredient. Fleas (Ctenocephalus felis) are effective within 8 hours after exposure, and ticks (Ixodes ricinus) are effective within 12 hours after exposure. Therefore, under extremely harsh conditions, the risk of disease transmission through parasites cannot be completely ruled out.
In addition to direct feeding, this product can be mixed into the dog food for feeding, and observe the dog during administration to confirm that the dog swallows the drug.
Withdrawal period:Need not be formulated
Package Strength:
1 tablet/box or 6 tablets/box
Adverse Reaction:
Very few dogs (1.6%) will have mild and transient gastrointestinal reactions, such as diarrhea, vomiting, loss of appetite, and salivation.
In 8-9 week-old puppies weighing 2.0-3.6 kg, were given 5 times the maximum recommended dose of fluralaner internally, once every 8 weeks, for a total of 3 times, and no adverse reactions were observed.
Oral administration of 3 times the maximum recommended dose of fluralaner in Beagles has not been found to have an effect on reproductive ability or survival of subsequent generations.
The Collie had multi-drug resistance gene deletion (MDR1-/-), and was well tolerated by internal administration of 3 times the maximum recommended dose of fluralaner, and no treatment-related clinical symptoms were observed.